Making the production of medicines against deadly diseases possible. The SDC supports.
For decades, an effective antidote to the deadly sleeping sickness lay dormant in the Sanofi archives. With the support of the SDC, the antidote was rediscovered by the "Drugs for Neglected Diseases Initiative" (DNDi) and a comparatively inexpensive, easy-to-administer and well-tolerated drug was developed.

Drugs for deadly diseases in the global South are not produced without the prospect of profit. This is where the SDC is trying to make a difference. © DNDi
What makes a product successful on the market? It has to solve a problem, in other words have value for customers. That is the demand side. In order for demand to be satisfied by a corresponding supply from private individuals, the product must generate a profit. However, it can happen that the development, production and distribution of the product do not pay off. For example, if customers can only pay very little or nothing at all. Then the market can fail. This phenomenon can be observed repeatedly in the case of diseases that affect millions of people in poorer countries. As a result, millions fall ill and die.
This is where the SDC wants to make a difference. Systemic market failure is to be corrected. The aim is not to replace private engagement, but to supplement it. Private, state, scientific and civil society engagement will be brought together to provide medicines for millions of people.
The Drugs for Neglected Diseases Initiative (DNDi) is a good example of how this works. This Geneva-based NGO has received CHF 24 million in funding from the SDC since it was founded in 2013. During this time, DNDi has supported the development of 13 treatments for deadly but neglected diseases. Millions of lives have been saved as a result.
Fexinidazole is one of these treatments. The search for a new cure for the deadly sleeping sickness began in 2005, when DNDi and the Swiss Tropical and Public Health Institute initiated a comprehensive search for active substances against parasites. They deliberately searched databases and archives for "forgotten" active substances. This led to the rediscovery of fexinidazole, whose preclinical development had been started by Hoechst (now Sanofi) in the 1970s but had never been completed. DNDi began further development in collaboration with Sanofi. The first clinical trials on trial subjects began in 2009, and by June 2019, fexinidazole was included in the World Health Organisation's (WHO) list of essential medicines for children and adults.
In between, more than two million people were tested for sleeping sickness in three clinical trials. The clinical trials included 749 patients in the Democratic Republic of Congo and the Central African Republic.
You must be able to afford falling ill
Being diagnosed with sleeping sickness used to mean weeks of hospitalisation at considerable cost. In addition, there was the loss of earnings during this time. Whole families quickly found themselves in existential distress. The newly developed drugs are not only easier to administer, but also no longer require hospitalisation.
But why was all this effort made? After all, Melarsopol, a drug against sleeping sickness, has been available since 1949. Although Melarsopol is effective in combating sleeping sickness, it has considerable side effects. And in 5-10 per cent of cases, the side effects are even fatal. Eflornithine, which has been available since the 1980s, is another effective drug against sleeping sickness. However, commercialisation in Africa is not financially viable for pharmaceutical companies. Transporting and storing the sensitive active ingredient is too costly. Administration is similarly costly. It requires 14 days in hospital and 56 intravenous infusions. In Europe, however, the active ingredient is sold in a cosmetic product to treat hair growth in women.
The story of sleeping sickness
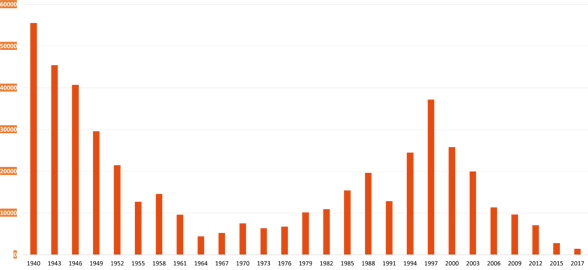
Finally, in March 2020, after more than ten years, the first patient was treated outside of a clinical trial in the Democratic Republic of the Congo. Unlike previous drugs, flexinidazole can be taken orally by patients in the form of a tablet. In addition, transport and storage are comparatively simple. This means that even remote regions can be supplied.
Today, the main task is to make fexinidazole actually accessible. Together with the WHO and the support of Sanofi, DNDi is driving forward the training and – just as importantly – the updating of national treatment and pharmaceutical monitoring guidelines in Angola, the Central African Republic, the Democratic Republic of Congo, Guinea and South Sudan.
If the project succeeds, it could be a decisive step towards eradicating the deadly disease.



